Particle.news
Health ❯ Healthcare ❯ Clinical Trials
FDA FDA Approval FDA Regulations Regulatory Affairs FDA Approvals NHS Treatments Surrogate Endpoints NHS Innovations Elinzanetant EMA Recommendations Alyftrek and Kaftrio Efficacy Studies Neurology NHS Guidelines Combination Therapy NHS ZORYVE Cream Survival Rates Blarcamesin Semaglutide FLOW Trial Kisqali EMA Eli Lilly Trials FDA Fast-Track Approval Therapeutic Goods Administration

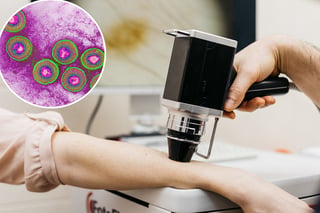
The decision rests on phase 2 data showing high response rates with labeling that highlights cytokine release syndrome and neurologic risks.