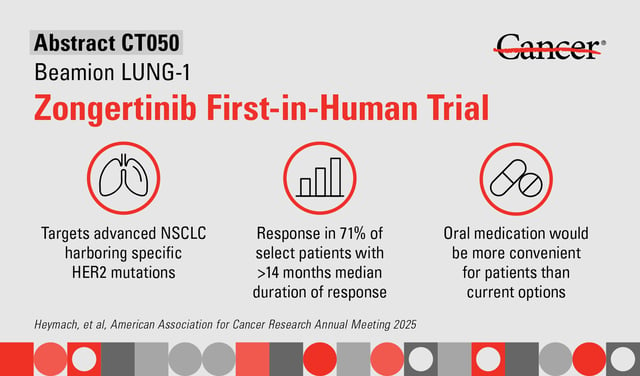
Overview
- Zongertinib demonstrated a 71% objective response rate, with a median duration of response of 14.1 months and progression-free survival of 12.4 months, in HER2-mutant NSCLC patients.
- The therapy is a once-daily oral HER2-selective tyrosine kinase inhibitor that spares EGFR, resulting in reduced adverse effects compared to less selective treatments.
- Grade three or higher adverse events occurred in only 17% of patients, with no reported cases of interstitial lung disease, a common concern with current treatments.
- The FDA granted zongertinib breakthrough therapy designation and priority review based on earlier trial results, and Phase II trials and first-line studies are now underway.
- Zongertinib offers a targeted and more convenient alternative to the intravenous antibody-drug conjugate trastuzumab deruxtecan, addressing significant unmet needs in this patient population.