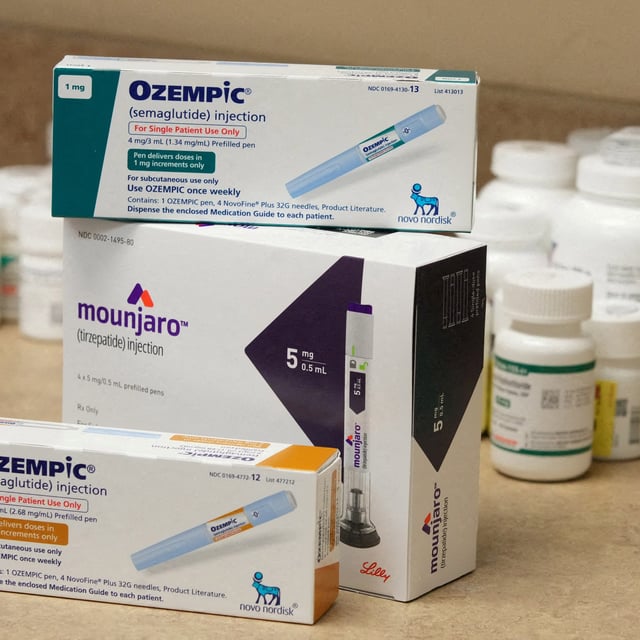
Overview
- The WHO limited the listing to type 2 diabetes patients with established cardiovascular disease, chronic kidney disease or obesity, excluding obesity as a stand‑alone indication.
- Officials said high prices restrict access and urged generic production as patents begin to expire, with Canada expected to see semaglutide generics in 2026.
- WHO leaders described the move as a catalyst intended to guide national coverage decisions and expand availability in public health systems.
- The update also added Vertex’s Trikafta/Kaftrio for cystic fibrosis, Merck’s Keytruda for selected advanced cancers, and rapid‑acting insulin analogues.
- The WHO cited the global burden of more than 800 million people with diabetes and over 1 billion with obesity as context for prioritizing these therapies.