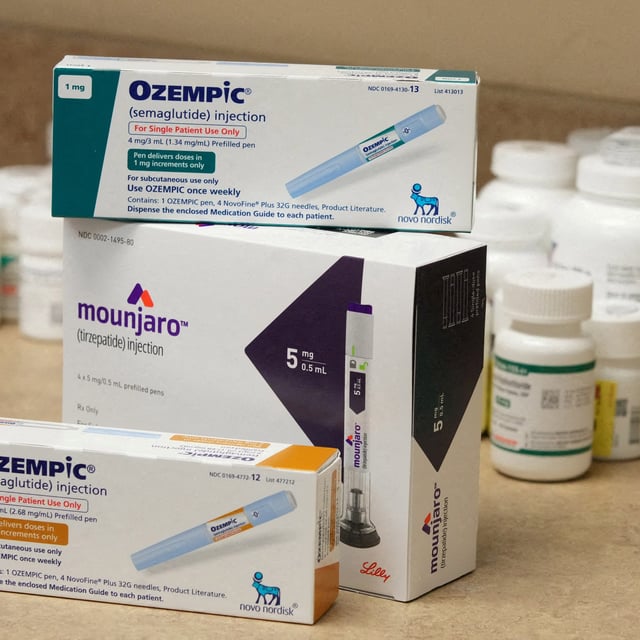
Overview
- GLP-1 receptor agonists—semaglutide, dulaglutide, liraglutide—and the dual GLP-1/GIP agent tirzepatide were listed for adults with type 2 diabetes plus established cardiovascular disease, chronic kidney disease or obesity.
- The committee did not add these medicines for obesity-only treatment, limiting use to diabetes with specific comorbidities.
- Other additions include pembrolizumab as first-line monotherapy for metastatic cervical, colorectal and non-small cell lung cancers, with atezolizumab and cemiplimab listed as alternatives for NSCLC.
- The update also adds the cystic fibrosis triple therapy elexacaftor/tezacaftor/ivacaftor and rapid-acting insulin analogues for type 1, type 2 and gestational diabetes.
- WHO cited high prices as a barrier and urged countries to prioritize high-need patients, expand primary-care availability and encourage generic competition as patents begin to expire, noting the lists now cover 523 adult and 374 pediatric medicines used by more than 150 countries to guide procurement.