Overview
- Phase 1 results showed all nine treatment-resistant PTSD patients achieved complete remission, remaining symptom-free for six months post-therapy.
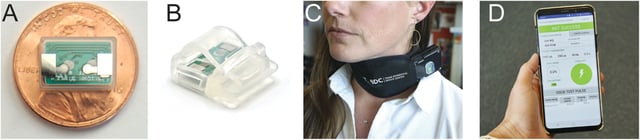
- The therapy combines prolonged exposure treatment with a miniaturized vagus nerve stimulation (VNS) device implanted in the neck.
- The VNS device, about the size of a dime, is wirelessly powered and controlled via a smartphone during therapy sessions.
- The ongoing Phase 2 trial in Dallas and Austin is double-blind and placebo-controlled, a key step toward potential FDA approval.
- Funded by DARPA, this research builds on prior successes using VNS for stroke rehabilitation, addressing a critical need for effective PTSD treatments.