Overview
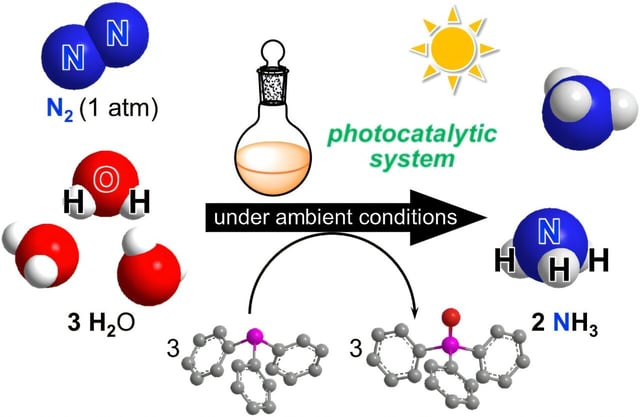
- The University of Tokyo team has scaled up ammonia synthesis using artificial photosynthesis to a volume ten times larger than previous experiments, signaling readiness for pilot trials.
- The process uses sunlight and dual molecular catalysts—iridium for water activation and molybdenum for nitrogen activation—to produce ammonia efficiently and with minimal energy input.
- This innovation offers a low-carbon alternative to the Haber-Bosch process, which accounts for about 2% of global CO₂ emissions and dominates current ammonia production.
- Challenges remain in addressing the potential toxicity of tertiary phosphines used in the process and improving the sustainability of the catalysts for long-term use.
- The findings, published in Nature Communications, highlight the potential for decentralized, small-scale ammonia production that could reduce transportation emissions and support clean energy applications.