Overview
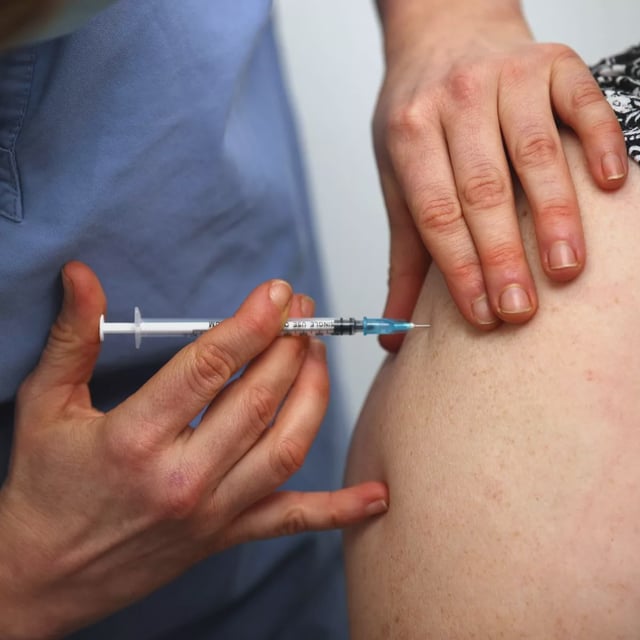
- The first-in-human trial for SPVX02, a fridge-free tetanus-diphtheria vaccine, is underway at NIHR Southampton under UK researchers Professor Saul Faust and Dr. Karen O’Hanlon.
- SPVX02 remains stable at room temperature and extreme conditions, from –20 °C to 40 °C, for at least a year, reducing reliance on cold storage.
- The trial aims to assess the vaccine’s safety and immunogenicity, with completion expected this summer and results anticipated by the end of 2025.
- The World Health Organization estimates up to 50% of vaccines are wasted annually due to cold-chain failures, which this innovation seeks to address.
- Stablepharma aims to expand the technology to other vaccines, with a global rollout of SPVX02 targeted for 2027, pending successful trial outcomes.