Overview
- In the SunRISe-1 trial of 85 high-risk non-muscle-invasive bladder cancer patients unresponsive to BCG, TAR-200 achieved complete tumor elimination in 82% of participants.
- Most participants experienced tumor disappearance within three months, and nearly half remained cancer-free at one year.
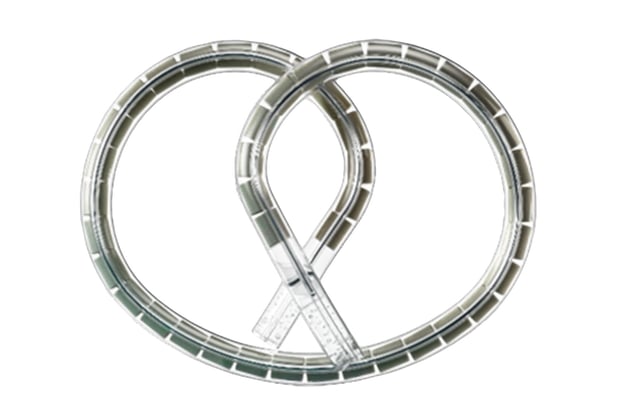
- The pretzel-shaped intravesical device delivers gemcitabine continuously over three-week cycles, extending drug exposure beyond standard instillations.
- Adding the immunotherapy cetrelimab to TAR-200 did not improve efficacy and resulted in more adverse effects.
- Johnson & Johnson’s New Drug Application for TAR-200 has been accepted for FDA priority review as the study closes enrollment and long-term follow-up proceeds.