Overview
- The Phase IIb SunRISe-1 trial enrolled 85 BCG-unresponsive high-risk non-muscle-invasive bladder cancer patients at 144 global sites and delivered TAR-200 every three weeks for six months followed by quarterly dosing.
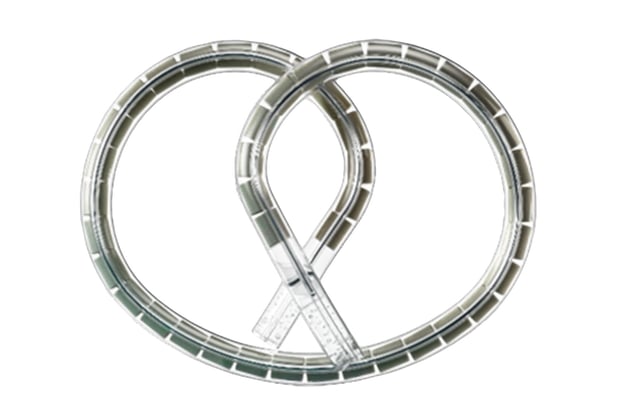
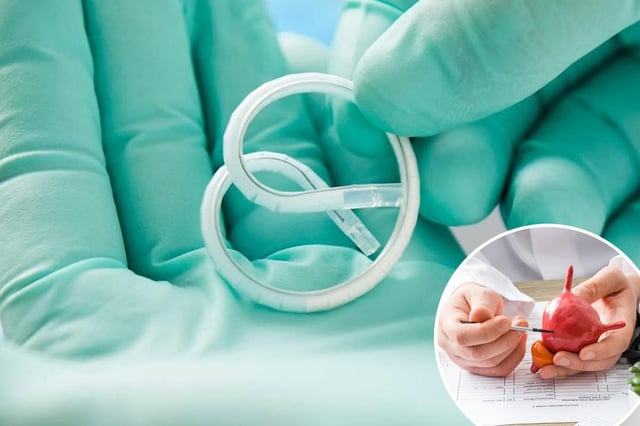
- The pretzel-shaped intravesical device provided continuous gemcitabine release over three weeks per cycle, extending drug contact time compared with conventional instillations.
- Seventy of 85 patients achieved complete tumor response at three months, and nearly half of those responders remained cancer-free at one year.
- TAR-200 monotherapy demonstrated a favorable safety profile with mostly mild, transient urinary symptoms, while the arm combining TAR-200 with cetrelimab showed lower response rates and increased side effects.
- The FDA has granted TAR-200 a New Drug Application Priority Review and enrolled patients will continue long-term follow-up as Johnson & Johnson advances toward regulatory approval and commercialization.