Overview
- Swissmedic granted fast-track authorization for Coartem Baby to treat infants weighing between 2 and 5 kilograms, making it the first approved antimalarial for this age group.
- The dissolvable, cherry-flavored formulation can be mixed with breast milk to simplify dosing and reduce overdose risks in newborns.
- Coartem Baby was developed by Novartis in collaboration with the Medicines for Malaria Venture and builds on the established artemether-lumefantrine therapy used in over a billion patients since 2000.
- Eight high-burden countries in sub-Saharan Africa are reviewing the dossier and are expected to authorize the not-for-profit rollout within approximately 90 days.
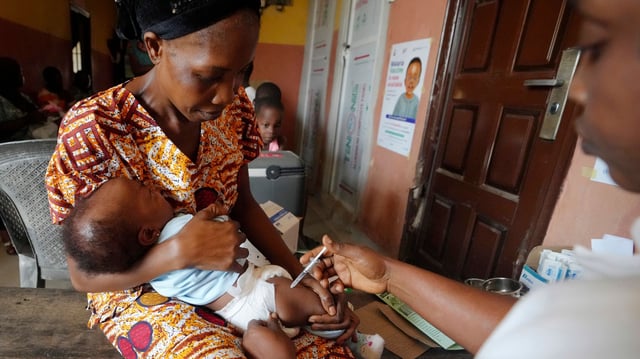
- The approval fills a critical gap that left infants under 4.5 kilograms—about 30 million born annually in malaria-endemic areas—without tailored, approved treatment.