Overview
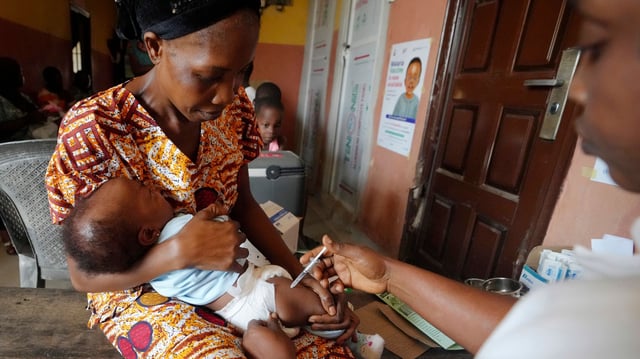
- Swissmedic granted fast-track authorization under its global health program for Coartem Baby, marking the first approved malaria drug for infants weighing 2–5 kg.
- Developed by Novartis in partnership with the Medicines for Malaria Venture, Coartem Baby emerged from a two-year trial that included newborns as young as one day old.
- The treatment is formulated to dissolve in breast milk and features a sweet cherry flavor to simplify dosing and improve caregiver compliance.
- Novartis will submit applications to regulators in Burkina Faso, Côte d’Ivoire, Kenya, Malawi, Mozambique, Nigeria, Tanzania and Uganda within 90 days based on Swissmedic’s findings.
- Rollout is expected to begin within weeks on a largely not-for-profit basis, aiming to close a critical treatment gap and reduce infant malaria mortality in high-burden African countries.