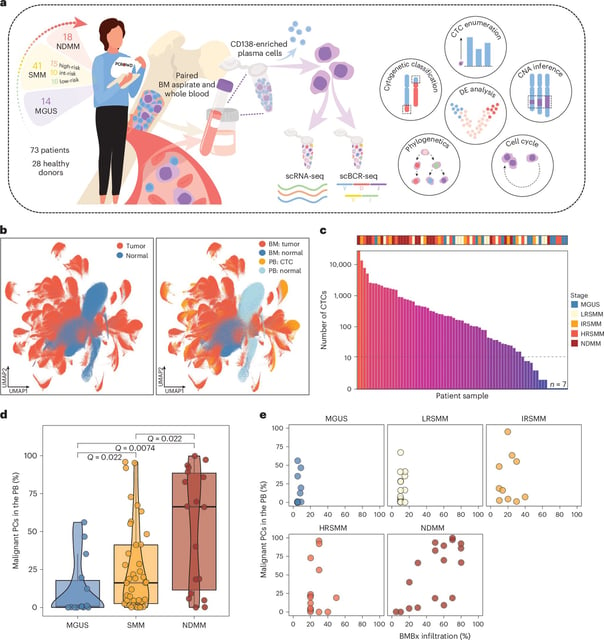
Overview
- SWIFT-seq captured circulating tumor cells in 90% of patients across MGUS, SMM and MM, including 95% of smoldering cases and 94% of newly diagnosed myeloma patients.
- The assay integrates single-cell enumeration, genomic alteration mapping, tumor proliferation assessment and prognostic gene signatures in one blood-based test.
- By using molecular barcodes rather than cell surface markers, SWIFT-seq delivers genomic profiles that outperform fluorescent in situ hybridization in accuracy.
- Researchers identified a novel gene signature linked to tumor circulatory capacity, offering fresh targets for studying myeloma spread and drug development.
- Successful validation in a 101-patient cohort positions SWIFT-seq for clinical integration and future therapeutic research.