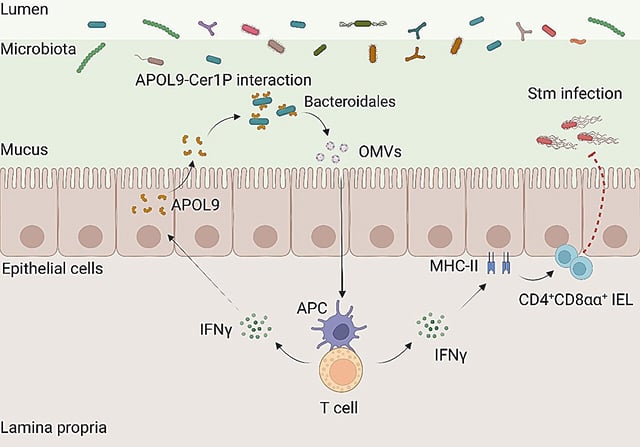
Overview
- APOL9, a host protein, selectively binds to ceramide-1-phosphate (Cer1P), a lipid found on Bacteroidales bacteria, enabling precise microbial targeting.
- Unlike traditional antimicrobial proteins, APOL9 induces the release of outer membrane vesicles (OMVs) from Bacteroidales rather than killing them.
- OMVs enhance mucosal immunity by boosting interferon-gamma (IFN-γ) signaling and increasing MHC-II expression, essential for training specialized gut T cells.
- Mice lacking APOL9 showed weaker immune responses to Salmonella infections, but administering OMVs restored immune function and reduced infection severity.
- Researchers are now investigating APOL9’s human homolog, APOL2, and exploring therapeutic strategies to modulate this lipid-based communication pathway.