Overview
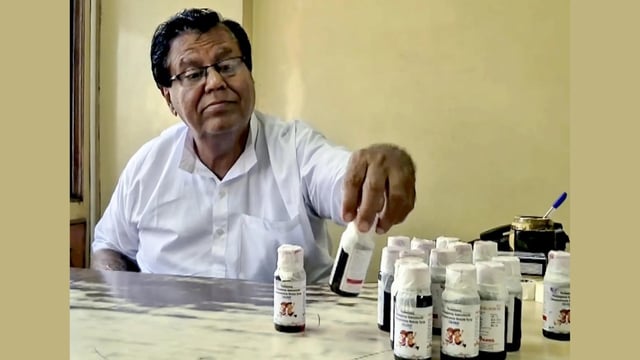
- The Telangana Drugs Control Administration issued a public alert for Coldrif Syrup batch SR-13, manufactured in May 2025 with expiry in April 2027, directing retailers and hospitals to freeze stocks and urging the public to stop use and report holdings.
- Kerala suspended the sale and distribution of the product as a precaution, with Health Minister Veena George saying inspectors have ordered stock removal and sent samples for testing even though the flagged batch was not found in the state.
- The Union Health Ministry announced risk-based inspections at manufacturing premises of 19 drugs across six states as part of a broader probe into reported child deaths linked to cough syrup use in Madhya Pradesh and Rajasthan.
- Tamil Nadu’s Food Safety and Drug Administration reported Coldrif samples from Sresan Pharma’s Kanchipuram facility contained diethylene glycol beyond permissible limits, and the state sealed the plant and banned sales with an immediate withdrawal of stocks.
- The Health Ministry said six samples tested by the CDSCO were free of diethylene glycol and ethylene glycol, and Madhya Pradesh officials reported some negatives as well, while a multidisciplinary team from national institutes continues to analyze samples and assess causes in Chhindwara.