Overview
- Tamil Nadu banned sales from October 1 and halted production at the Kanchipuram unit, Madhya Pradesh ordered a statewide ban and seizures, Kerala suspended sales, and Telangana issued a stop‑use alert for Batch SR‑13.
- Authorities reported child deaths linked to cough syrups in Madhya Pradesh’s Chhindwara cluster and in Rajasthan, with MP counts reported between 11 and 14 across outlets.
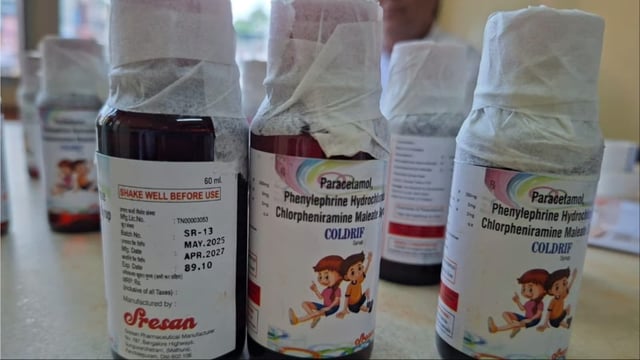
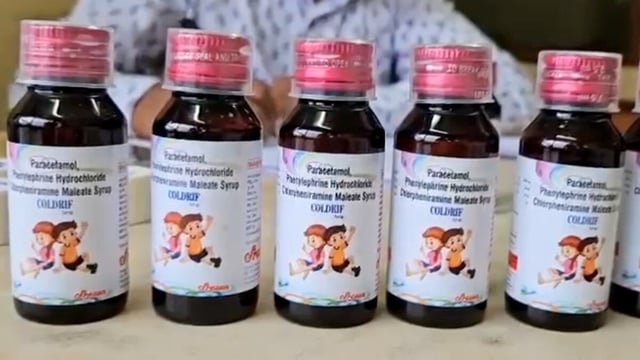
- CDSCO and MPFDA said initial tests on other collected samples showed no DEG/EG, while Tamil Nadu’s Drug Testing Laboratory detected 48.6% DEG in Coldrif Batch SR‑13 (Mfg May 2025; Exp Apr 2027).
- Police registered cases against the manufacturer and arrested a local doctor who prescribed the syrup as criminal and regulatory investigations widen.
- CDSCO launched risk‑based inspections of units in Himachal Pradesh, Uttarakhand, Gujarat, Tamil Nadu, Madhya Pradesh and Maharashtra, as expert teams analyse samples and national advisories restrict cough and cold medicines for very young children.