Overview
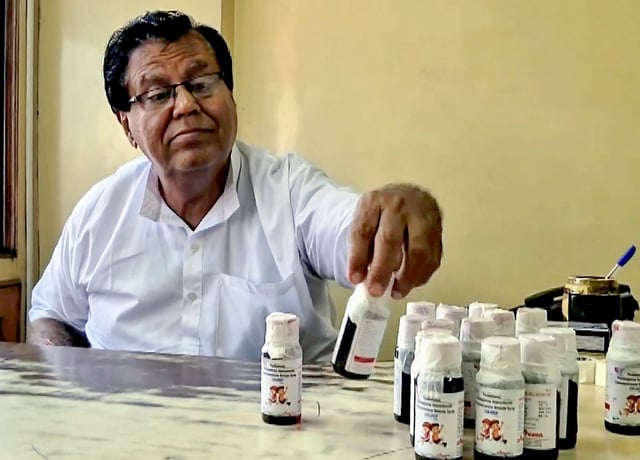
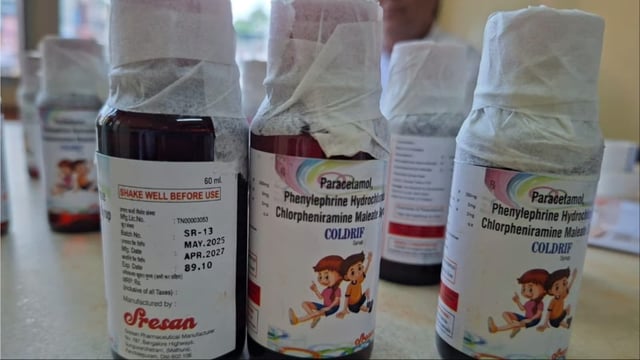
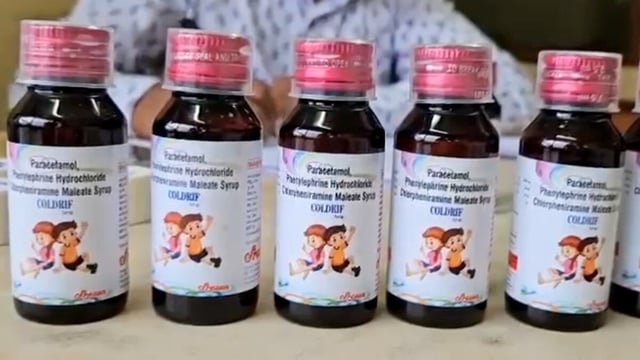
- Tamil Nadu’s Drug Testing Laboratory declared Coldrif batch SR-13 (Mfg May 2025, Exp Apr 2027) adulterated, reporting 48.6% diethylene glycol and branding it not of standard quality.
- Madhya Pradesh banned and began seizing Coldrif and suspended other products from manufacturer Sresan Pharmaceuticals; Tamil Nadu and Kerala halted sales, and Telangana issued a stop‑use public alert.
- Madhya Pradesh police registered an FIR against Sresan Pharmaceuticals and arrested paediatrician Praveen Soni in Chhindwara after at least 11 child deaths linked to acute kidney injury.
- The Union Health Ministry launched risk‑based inspections of drug manufacturing units across six states as CDSCO collected samples of 19 medicines for testing.
- A multi‑agency team from NIV, ICMR, NEERI, CDSCO and AIIMS‑Nagpur is analysing clinical and product samples, with officials noting some earlier CDSCO and state tests on other drugs showed no DEG/EG contamination.