Overview
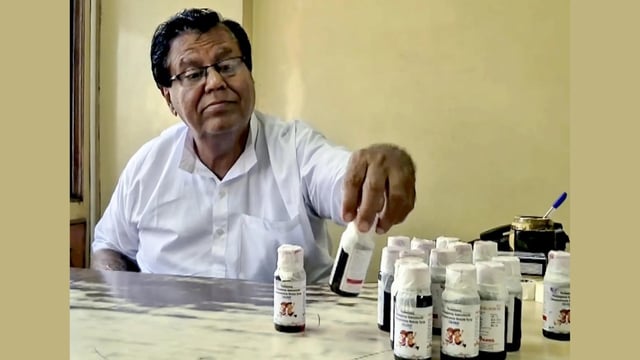
- Tamil Nadu testing of Coldrif batch SR-13 from Sresan Pharma’s Kanchipuram unit detected diethylene glycol above limits, with a Chennai government lab report citing 48.6% DEG.
- Madhya Pradesh, Tamil Nadu and Kerala halted sales, Telangana issued a public alert to freeze stocks, and production at the Kanchipuram plant was stopped pending further orders.
- CDSCO began risk-based inspections of manufacturers tied to 19 sampled drugs across Himachal Pradesh, Uttarakhand, Gujarat, Tamil Nadu, Madhya Pradesh and Maharashtra.
- Authorities are investigating the deaths of children reported in Madhya Pradesh and Rajasthan, with at least 11 fatalities cited, as a multidisciplinary team analyzes samples and clinical factors.
- Some samples tested by CDSCO and the MP FDA were negative for DEG/EG and were not from the two suspected syrups, while Madhya Pradesh announced Rs 4 lakh ex gratia for each deceased child’s family.