Overview
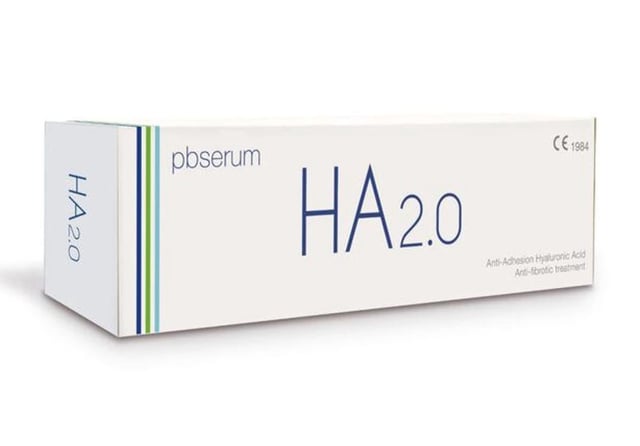
- The Spanish Agency for Medicines and Medical Devices (AEMPS) has banned the commercialization of HA Corrector, Pbserum Enzymatic Solution, and Pbserum HA.
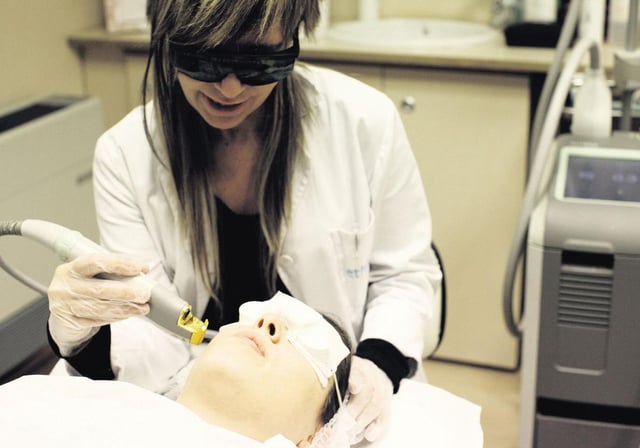
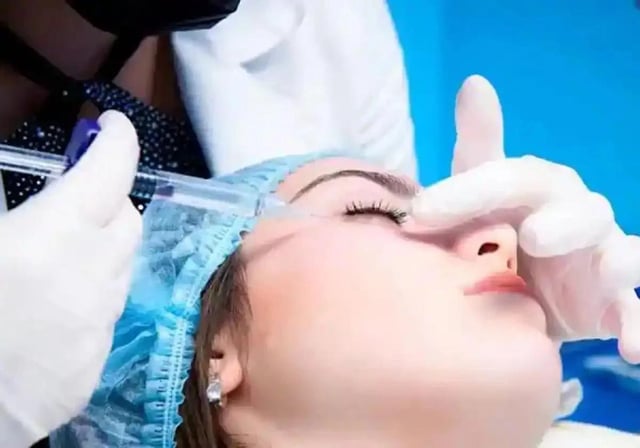
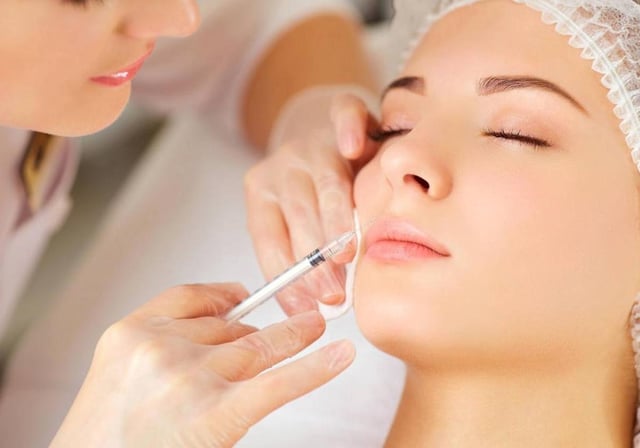
- These products, classified as cosmetics, were improperly promoted for injection to address complications from hyaluronic acid fillers.
- Hyaluronidase, an enzyme included in these products, is prohibited for use in cosmetics under Spanish law.
- AEMPS has instructed the immediate market withdrawal and recovery of all affected units, citing unproven safety and efficacy for aesthetic purposes.
- Consumers are advised not to use these products and to return them to the point of purchase or contact the manufacturer for further guidance.