Overview
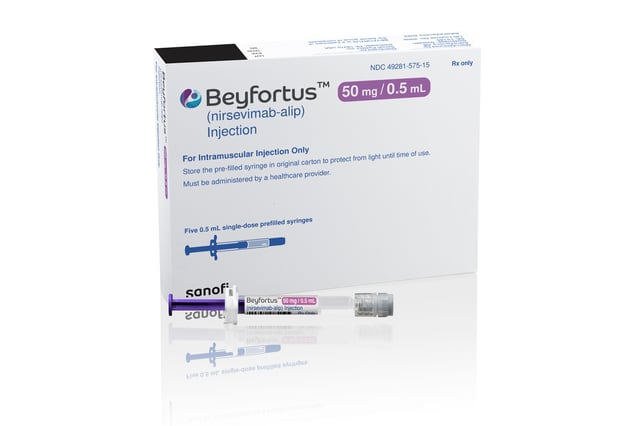
- Due to high demand, Sanofi's new respiratory syncytial virus (RSV) preventative drug, Beyfortus, is experiencing a supply shortage. This has led the CDC to recommend priority use for infants at highest risk.
- RSV is a leading cause of infant hospitalizations worldwide, and Beyfortus is the first preventative drug of its kind on the market, resulting in high uptake since its launch.
- Some children have been unable to receive Beyfortus due to the shortage, revealing deeper structural issues with the US childhood vaccination system and concerns surrounding insurance reimbursement.
- Sanofi had assured that there would be no supply problem; thus the shortage has been frustrating for health professionals. Other obstacles, such as the way vaccines are paid for in the US, are likely to create further challenges for Beyfortus access.
- RSV causes 58,000 hospitalizations and up to 300 deaths each year in the US. Babies up to 19 months who were born prematurely or have underlying conditions are at risk for severe RSV disease, yet due to an immature immune system, these babies struggle to respond well to vaccines. Beyfortus thus presents a breakthrough in child health as it effectively protects them from a severe RSV infection.