Overview
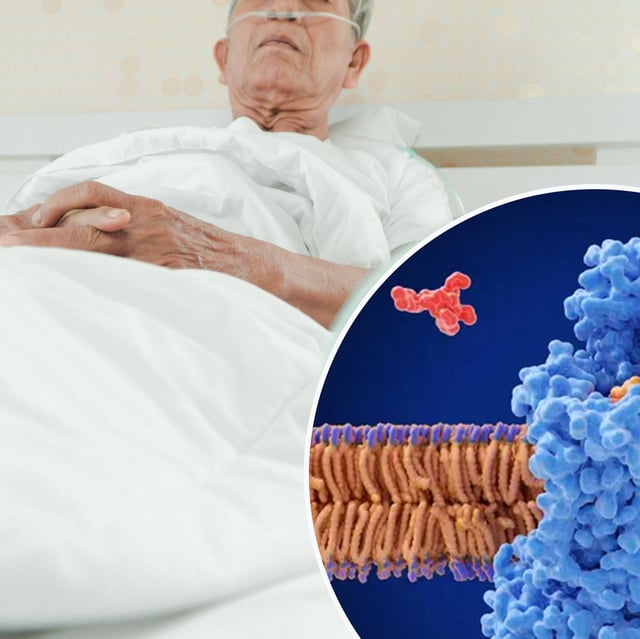
- Zosurabalpin is the first antibiotic in over five decades to show promise against carbapenem-resistant Acinetobacter baumannii, a bacteria the WHO lists as a priority pathogen.
- It employs a novel mechanism that interferes with the formation of the bacterium’s outer membrane, overcoming a key barrier to existing treatments.
- The Phase III trial is slated to begin later this year or in early 2026 and will randomize approximately 400 patients to receive either zosurabalpin or the current standard of care.
- If the trial succeeds, Roche aims to seek regulatory approval by the end of the decade, potentially filling a critical gap in hospital-acquired pneumonia and sepsis therapies.
- Experts warn that antimicrobial resistance could cause up to 10 million deaths annually by 2050, underscoring the urgent need for new antibiotics.