Overview
- Researchers at the University of British Columbia screened over 240 FDA-approved compounds against TB-infected immune cells and pinpointed benztropine as a host-directed lead.
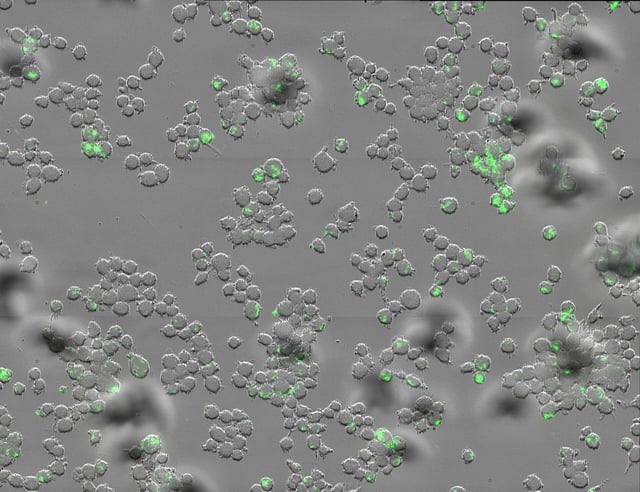
- Benztropine inhibits a histamine receptor on macrophages that Mycobacterium tuberculosis exploits, restoring the cells’ ability to kill the bacteria.
- Lab assays with human and mouse macrophages showed dramatic bacterial reductions and oral treatment cut lung bacterial loads by about 70 percent in TB-infected mice.
- Because benztropine is already approved for Parkinson’s disease, its established safety profile could speed initiation of clinical trials for TB.
- Investigators stress that human studies are still required to establish effective dosing, confirm safety in TB patients and explore combination use with existing antibiotics.