Overview
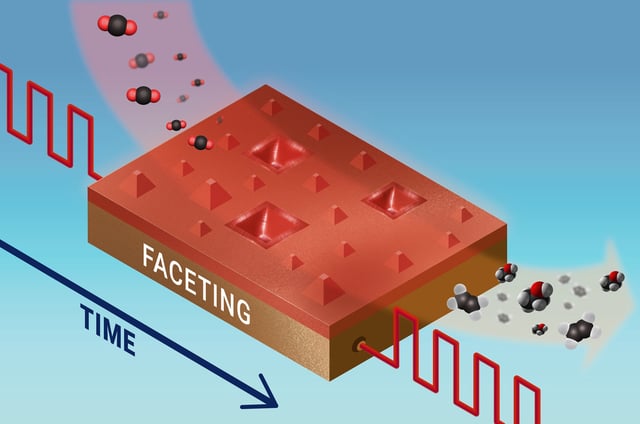
- The Nature Catalysis study reports that alternating anodic (~+0.6 V) and cathodic (~−1 V) pulses reshape and partially oxidize Cu(100), boosting CO2 electroreduction performance.
- Inverted-pyramid faceting forms during oxidizing pulses via site-selective dissolution, creating specific side facets linked to product outcomes.
- Following reduction pulses, the surface adopts a sandwich-like near-surface with ~0.5 nm metallic Cu over ~0.5 nm Cu(I), which emerges from partial reduction of an oxidized film.
- Correlated LEEM/XPEEM spectro-microscopy directly connects these morphological and chemical-state changes to shifts in selectivity between ethanol and ethylene.
- The Fritz Haber Institute team demonstrates tunability on single-crystal model electrodes after short pulsed CO2RR runs, with practical durability and scale-up still untested.