Overview
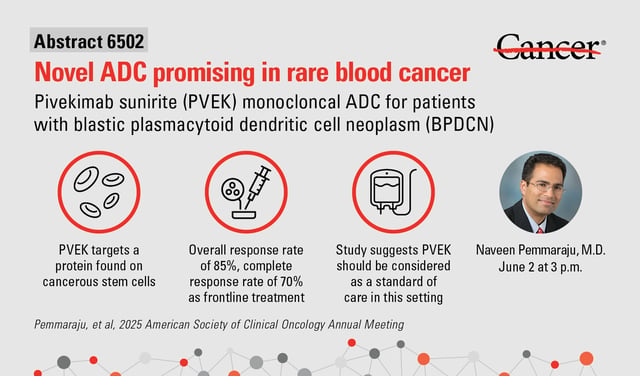
- The multi-center Phase I/II CADENZA trial enrolled 84 BPDCN patients receiving PVEK every 21 days in both frontline and relapsed or refractory settings.
- Frontline participants achieved an 85% overall response rate, with 70% attaining complete remissions.
- PVEK employs a monoclonal antibody linked to a cytotoxic payload to selectively target CD123-expressing BPDCN cells.
- The most common side effect was reversible peripheral edema, offering a more manageable safety profile compared with capillary leak syndrome associated with tagraxofusp-erzs.
- Led by MD Anderson researchers and funded by AbbVie, the study paves the way for PVEK’s exploration in combination therapies and potential adoption as a new treatment standard.