Overview
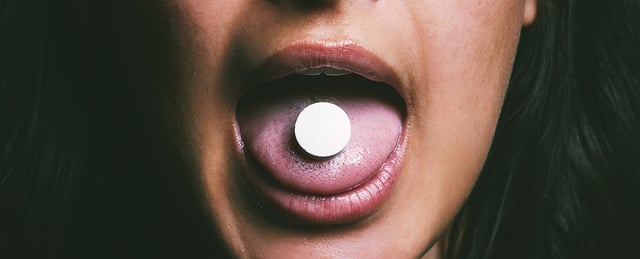
- A once-daily 25 mg oral semaglutide pill produced a mean 13.6% weight reduction versus placebo in a 64–71 week trial, with 16.6% average loss among adherent participants and 34.4% achieving at least 20% weight loss.
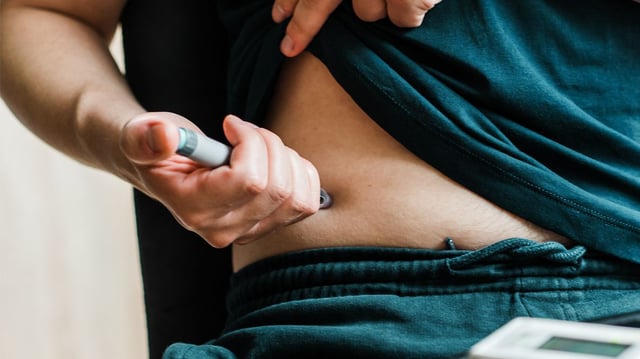
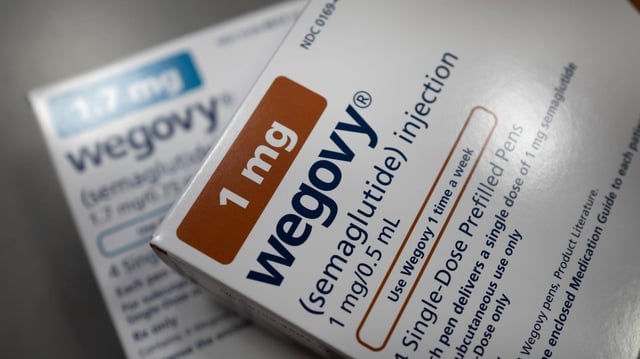
- In adults with obesity and type 2 diabetes, a 7.2 mg weekly injection cut body weight by 13.2% over 72 weeks versus 10.4% with the approved 2.4 mg dose and 3.9% with placebo, and delivered larger waist reductions.
- Gastrointestinal symptoms were frequent with the oral pill (74% versus 42.2% on placebo), while the 7.2 mg injection showed higher dysesthesia rates (18.9% versus 4.9% at 2.4 mg) and more dose reductions, with one death possibly related to the drug.
- Novo Nordisk has filed a U.S. application for the oral formulation, with an FDA decision expected by year-end 2025, and says a pill could broaden access for patients who avoid injections or lack refrigeration.
- Both studies were company-funded; the oral trial excluded people with diabetes, and independent experts are calling for longer-term safety data and clearer measures of fat versus lean mass changes.