Overview
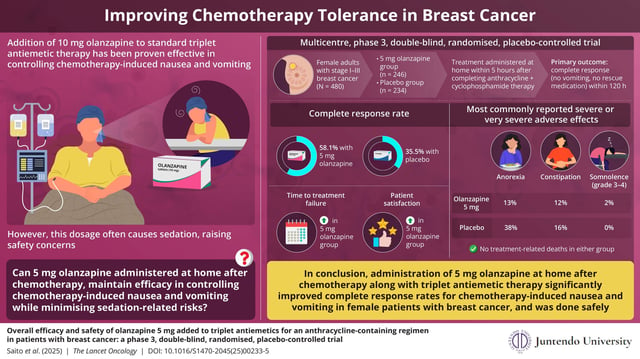
- A phase 3, double-blind, placebo-controlled trial of 500 Japanese breast cancer patients found 58.1% of those receiving 5 mg olanzapine achieved complete response in the first five days after anthracycline-plus-cyclophosphamide chemotherapy versus 35.5% on placebo.
- A single 5 mg dose administered at home within five hours post-chemotherapy and before the evening meal delivered effective antiemetic control while minimizing sedation.
- Severe or very severe concentration impairment occurred in 10% of patients on 5 mg olanzapine compared with 14% on placebo, and no treatment-related deaths were reported.
- The trial’s patient-centered design was shaped by feedback from breast cancer survivors at a 2015 MASCC meeting to reduce burdensome side effects and support outpatient care.
- By cutting sedation risks and financial burden compared with the standard 10 mg dose, the low-dose regimen could improve access to supportive care in resource-limited settings.