Overview
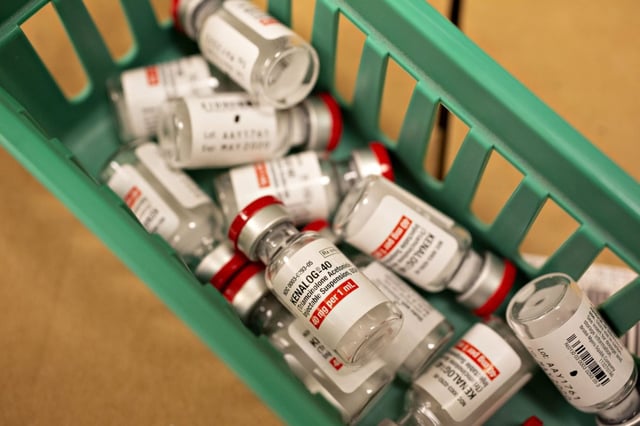
- The National Pharmacy Association (NPA) has reported a 96% increase in hay fever-related queries at pharmacies since April, with 45% receiving questions about the unlicensed steroid injection Kenalog.
- Kenalog, a prescription-only steroid not licensed for hay fever in the UK, was withdrawn from NHS use a decade ago due to severe side effects, including vision loss, high blood pressure, and depression.
- The NPA warns that unregulated sellers, including online platforms and beauty salons, may offer counterfeit or unsafe versions of Kenalog, posing significant health risks to patients.
- Regulators are being urged to reinstate public listings of approved online medicine sellers, a measure previously in place under EU regulations but removed post-Brexit.
- Pharmacists recommend safe, licensed alternatives such as antihistamines and steroid nasal sprays for managing hay fever symptoms, emphasizing the importance of consulting qualified professionals.