Overview
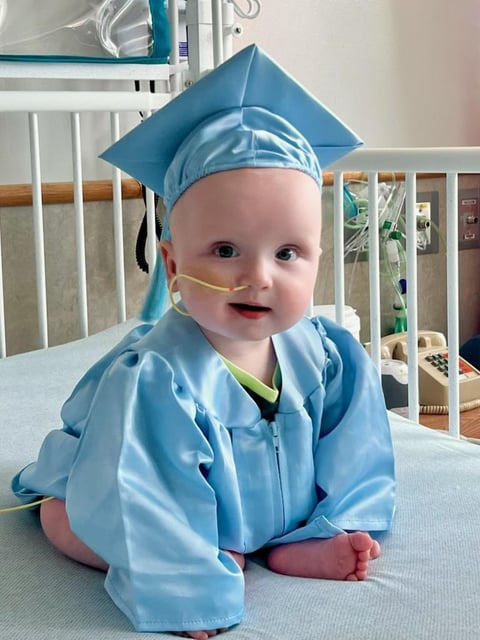
- KJ Muldoon, a 10-month-old with severe carbamoyl phosphate synthetase 1 deficiency, went home from Children’s Hospital of Philadelphia on June 3 after a 307-day stay.
- A multidisciplinary team at CHOP, the University of Pennsylvania and partner institutes designed a bespoke CRISPR-Cas9 base editor in six months to target his unique genetic mutation.
- Following two lipid nanoparticle infusions at seven and eight months, KJ’s ammonia levels stabilized, allowing higher protein intake and lower medication doses.
- His treatment was detailed in the New England Journal of Medicine and presented at the American Society for Gene and Cell Therapy in May.
- Partners at CHOP, Penn, the Innovative Genomics Institute and Danaher secured FDA approval within one week and are developing similar bespoke therapies for other ultra-rare disorders.