Overview
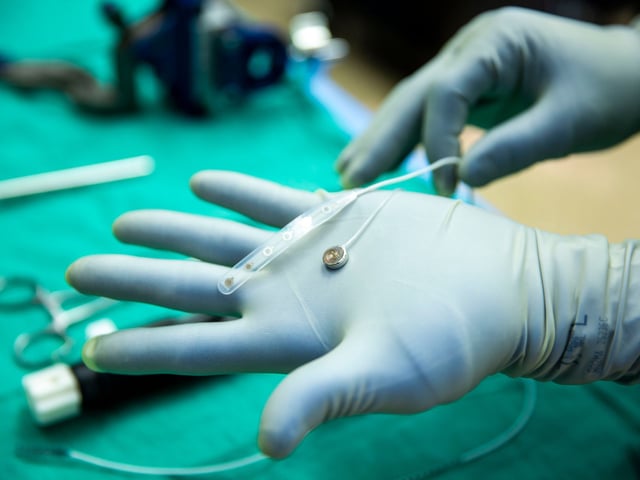
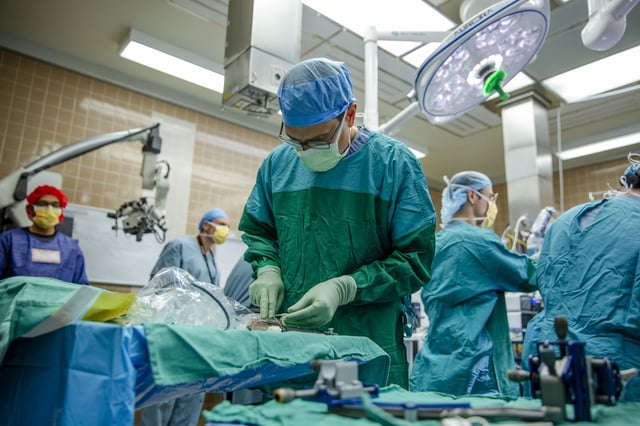
- Surgeons inserted and removed the dime-sized Connexus implant during a May 14 epilepsy surgery, marking its first human use.
- Over 420 microelectrodes recorded neural signals at the level of individual neurons, aiming to deliver higher-resolution data than existing BCI systems.
- Paradromics now enters the clinical stage and intends to launch long-term safety and efficacy trials of the Connexus BCI later in 2025, pending FDA approval.
- The startup has raised nearly $100 million in funding and secured a strategic partnership with Saudi Arabia’s Neom project to accelerate its neurotech development.
- Paradromics joins competitors such as Neuralink, Synchron and Precision Neuroscience in the race to commercialize implantable brain interfaces.