Overview
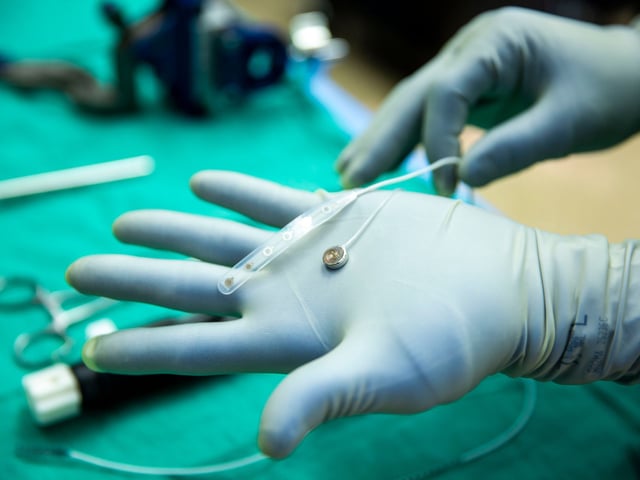
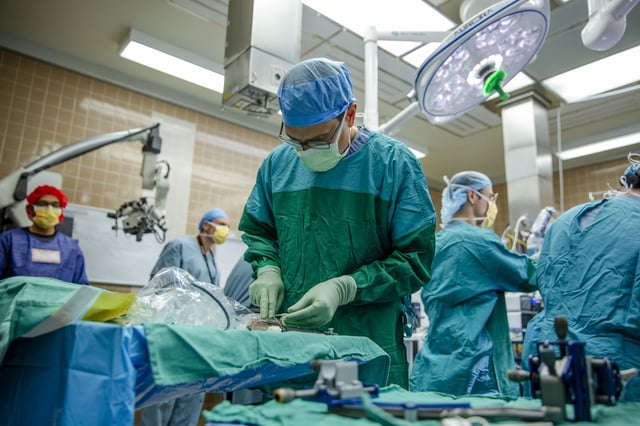
- The Connexus implant was placed and removed in about 20 minutes during a May 14 University of Michigan epilepsy surgery, successfully capturing neural activity without adverse effects.
- The fully implantable system uses 420 microelectrodes pushed into brain tissue and wirelessly transmits data to an under-the-skin transceiver.
- Paradromics will seek regulatory approval to launch a clinical trial later this year to study the device’s long-term safety and efficacy in humans.
- The company aims to decode neural signals into synthesized speech, text and cursor control to restore communication for patients with paralysis from ALS, stroke or spinal cord injury.
- This milestone advances Paradromics into the clinical stage alongside competitors Neuralink, Synchron and Precision Neuroscience in the race to commercialize high-resolution BCIs.