Overview
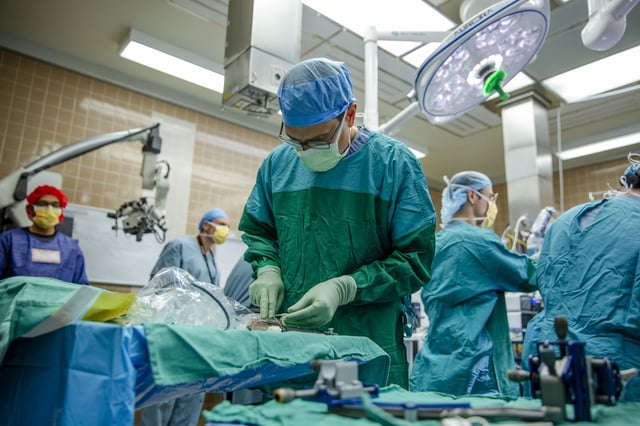
- On May 14, surgeons at the University of Michigan temporarily inserted and removed the dime-sized Connexus device during an epilepsy operation, confirming its ability to record electrical signals from the brain.
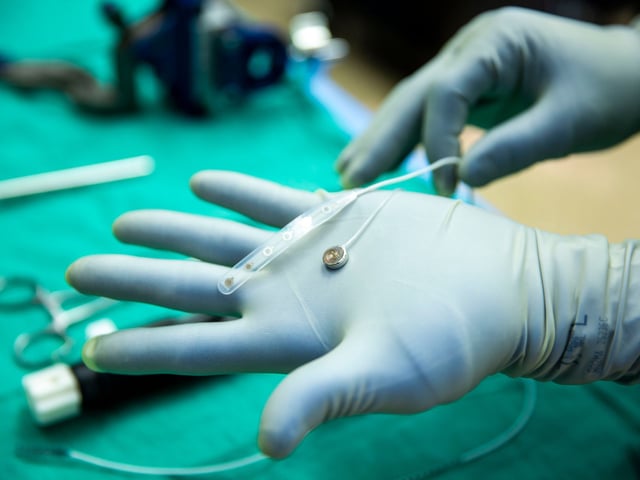
- The implant’s 420 micro-needle electrodes are designed to decode neural activity into synthesized speech, text and cursor control for patients with paralysis, stroke or ALS.
- Paradromics deployed a specialized EpiPen-like applicator to place the device through an existing craniotomy, minimizing incremental risk during the roughly 10-minute human test.
- This first human implant advances the nearly 10-year-old startup’s shift to clinical-stage development in a field that also includes Neuralink, Synchron and Precision Neuroscience.
- Paradromics has raised nearly $100 million and formed a strategic partnership with Saudi Arabia’s Neom, though the Connexus system still requires FDA clearance before broader trials and commercialization.