Overview
- Researchers built the cyclo[48]carbon catenane by linking three macrocycle-threaded polyyne chains and removing cobalt caps to unleash the protected 48-carbon ring.
- Convergent spectroscopic techniques—mass spectrometry, UV–visible, Raman spectroscopy and a single intense 13C NMR resonance—confirmed that all 48 sp1 carbons occupy equivalent environments in the catenane.
- An unprotected cyclo[48]carbon variant was detected at low concentration with a one-hour half-life at 20 °C and a UV–visible spectrum matching the protected form.
- This experiment constitutes only the second molecular carbon allotrope synthesized for study under normal laboratory conditions since the discovery of fullerenes in 1990.
- Researchers plan to crystallize the catenane for X-ray diffraction and probe its chemical reactivity and electronic properties.
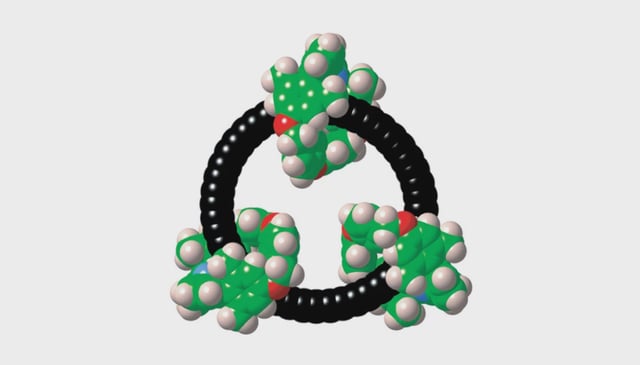
![Left: Chemical structure of the cyclo[48]carbon [4]catenane. RIGHT: Space-filling representation.](/cdn-cgi/image/onerror=redirect,width=640,height=640,format=webp/https://storage.googleapis.com/uploads.mongoosehq.com/url/media/39553022/6c3674fcd473fd05642db44e4276e64826a8d1749d712d43258bd4df826e6736)