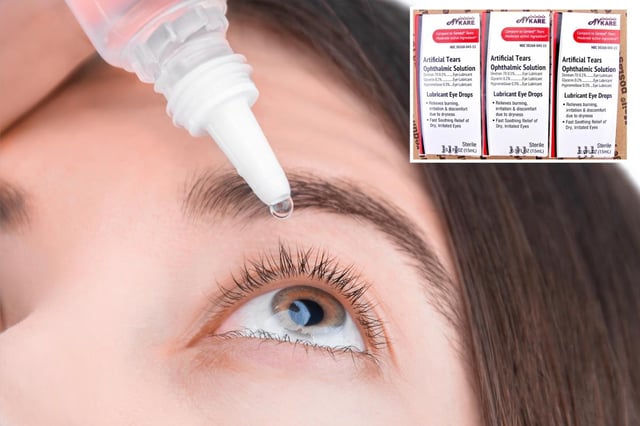
Overview
- The recall involves five specific ophthalmic products shipped between May 26, 2023, and April 21, 2025, including Artificial Tears and other formulations.
- An FDA audit identified deviations from Current Good Manufacturing Practice (cGMP) standards and a lack of sterility assurance in the recalled products.
- Consumers are advised to immediately discontinue use of the affected products and return them to AvKare for a full refund, including shipping costs.
- AvKare stated that the health risks associated with the recalled eye drops remain unknown, but potential patient safety concerns cannot be ruled out.
- This recall is part of broader FDA efforts to address sterility and quality issues in eye care products, reflecting increased regulatory scrutiny of manufacturing practices.