Overview
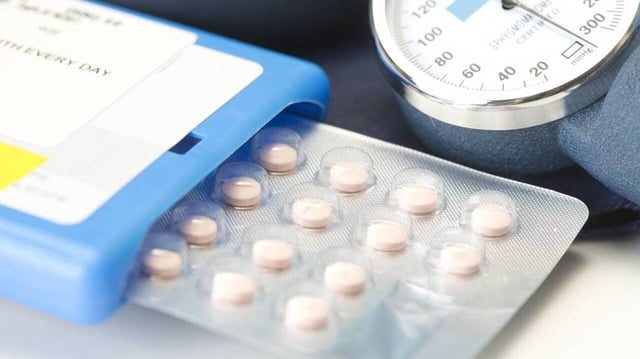
- The recall affects 32,640 boxes of Lisinopril in four specific 5 mg lots distributed by Viatris in France.
- A printing defect on the blister-pack aluminum could lead patients to mistakenly take multiple pills on certain days.
- The ANSM confirmed the therapeutic quality of the tablets and reported one non-serious overdose incident involving confusion.
- Symptoms of potential overdose include fatigue, balance issues, low blood pressure, and possible renal function impairment.
- Supply shortages are not expected, as alternative generics and compliant new lots will ensure continued availability.