Overview
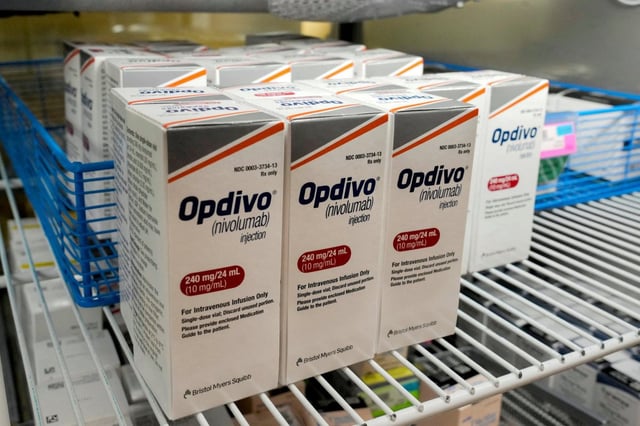
- The Medicines and Healthcare products Regulatory Agency (MHRA) has approved a new injectable form of the immunotherapy drug nivolumab, also known as Opdivo.
- This under-the-skin injection, which takes just 3–5 minutes to administer, will replace 30–60 minute intravenous infusions for eligible patients.
- The rollout, starting in May 2025, will make the NHS the first health service in Europe to offer this innovation, benefiting around 1,200 patients per month.
- The switch to the injectable form is expected to save approximately 1,000 hours of treatment time for patients and clinicians each month, improving hospital capacity.
- Thanks to a procurement agreement, the NHS will provide this treatment at no additional cost, enhancing efficiency while maintaining affordability.