Overview
- The systematic review consolidated 112 iBCI studies since 2020 and established the first global registry mapping 80 implanted participants across the United States, Europe, China and Australia.
- Fewer than 18 percent of trials measure patient-centered clinical outcomes, revealing a disparity between performance metrics and therapeutic assessments.
- The registry maps implant locations and device types and invites feedback from researchers to refine trial designs and standardize outcome reporting.
- Researchers emphasize harmonized outcome measures as a path to accelerate clinical translation of iBCIs for motor and speech restoration in people with paralysis.
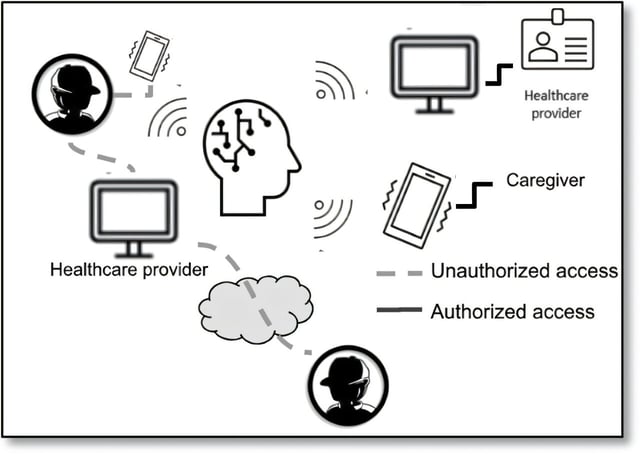
- A separate Yale-led study warns of cybersecurity risks in next-generation BCIs and recommends non-surgical software updates, robust authentication schemes and data encryption to protect patient safety and privacy.