Overview
- A retrospective study from Washington University School of Medicine analyzed 234 early-stage Alzheimer's patients treated with lecanemab and found severe side effects in only 1% of cases.
- The study highlights that patients with very mild symptoms experience the least risk, with only 1.8% developing any adverse symptoms compared to 27% of those with mild symptoms.
- Lecanemab, approved by the FDA in 2023, is the first treatment to slow Alzheimer's progression by targeting amyloid plaques, extending independent living by an average of 10 months.
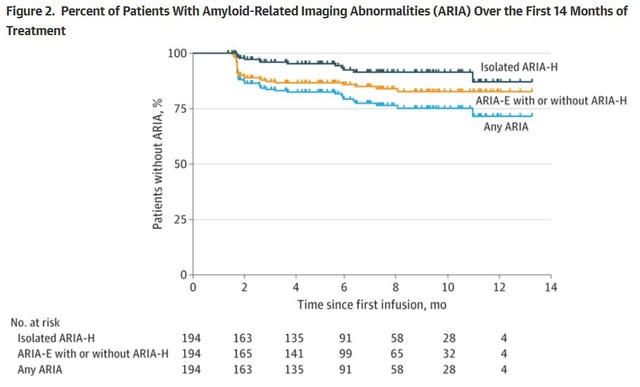
- Clinical trial findings on amyloid-related imaging abnormalities (ARIA) are consistent with real-world data, showing most cases are asymptomatic and resolve without intervention.
- Specialized memory clinics, like WashU Medicine’s Memory Diagnostic Center, play a critical role in safely administering lecanemab and monitoring patients with advanced imaging protocols.