Overview
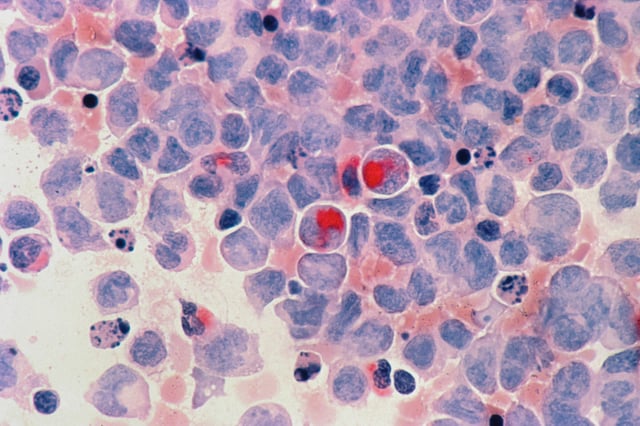
- The molecular bone marrow test identifies minimal residual disease (MRD) in acute myeloid leukemia (AML) patients up to three months before traditional blood tests detect relapse.
- A trial involving 637 patients in remission showed that quarterly bone marrow monitoring doubled long-term survival rates for those with NPM1 and FLT3 mutations.
- The 10-minute procedure, performed under local anesthesia, allows for early treatment intervention while patients remain well, reducing emergency hospitalizations.
- Results published in The Lancet Haematology have prompted the NHS to begin integrating the test into routine AML care across the UK, with researchers exploring global adoption.
- The test exemplifies advancements in precision medicine, with potential applications in other cancers and broader implications for early disease detection.