Overview
- The PRODI Center and betaSENSE have identified misfolded alpha-synuclein in cerebrospinal fluid as a reliable biomarker for early Parkinson's diagnosis.
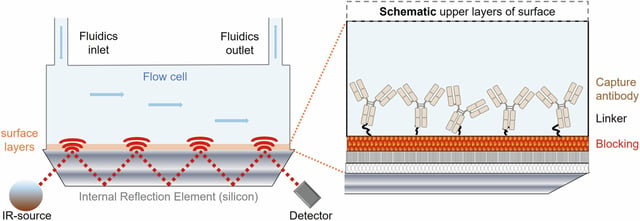
- The patented immuno-infrared sensor (iRS) technology directly detects protein misfolding, achieving sensitivity and specificity metrics exceeding 90%.
- Findings were validated in two independent clinical cohorts totaling 134 participants from research centers in Bochum and Kassel, Germany.
- The biomarker offers potential for therapeutic monitoring, enabling researchers to measure treatment effects in clinical trials targeting Parkinson's disease.
- Results, published in EMBO Molecular Medicine on April 25, 2025, mark a significant step toward integrating this diagnostic tool into clinical workflows.