Overview
- The UK’s Medicines and Healthcare products Regulatory Agency has approved Neuralink’s first European clinical study to enroll seven paralyzed patients in a trial of its N1 brain-computer interface.
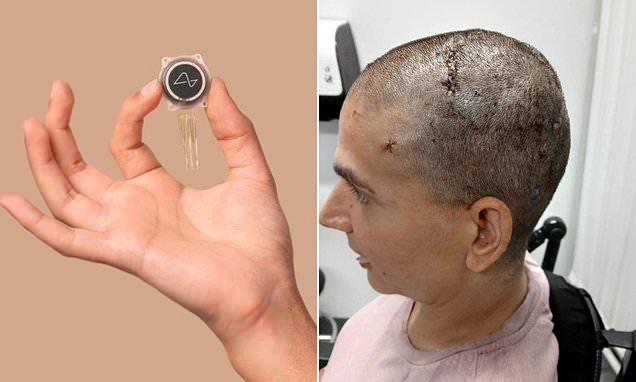
- The N1 device, roughly the size of a 10-pence coin, uses 128 ultra-thin threads and about 1,000 electrodes to translate neural activity into digital commands on smartphones and tablets.
- Neuralink will partner with University College London Hospitals and Newcastle upon Tyne Hospitals NHS trusts and employ its R1 surgical robot for precise chip implantation.
- Software updates developed after US trials preserved functionality when approximately 85 percent of the chip’s threads lost connection in early participants.
- The UK study represents Neuralink’s expansion beyond North America as it advances toward wider human-AI interface applications and future cognitive enhancements.