Overview
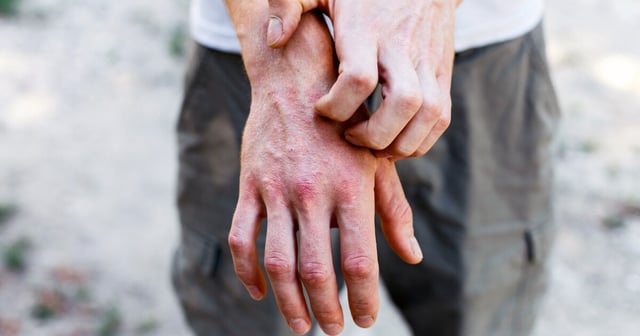
- The global Phase 2b REZOLVE-AD trial enrolled 393 patients with moderate-to-severe atopic dermatitis and delivered three subcutaneous doses of rezpegaldesleukin over 16 weeks.
- The treatment significantly improved the Eczema Area and Severity Index and produced rapid itch relief without raising rates of conjunctivitis or oral ulcers common with some biologics.
- Investors bid Nektar’s shares up 150% on June 24, sending its market value well above the pre-trial $250 million and highlighting confidence in the drug’s commercial prospects.
- With about 31.6 million Americans affected by atopic dermatitis and some patients unresponsive to existing therapies, rezpegaldesleukin addresses a sizable unmet need.
- The trial’s success has drawn acquisition interest from larger pharmaceutical firms and set the stage for rezpegaldesleukin to target peak annual sales over $2 billion if approved.