Overview
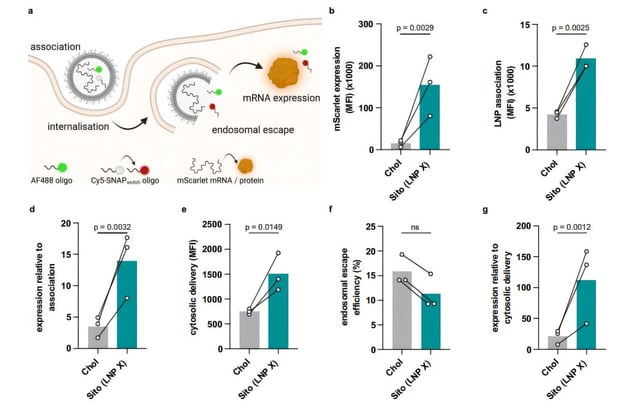
- Researchers at the Peter Doherty Institute in Melbourne developed a lipid nanoparticle, LNP X, that can ferry mRNA into resting CD4+ T cells with more than 75% efficiency without triggering toxicity or immune activation.
- The delivered mRNA instructs cells to produce the first 72 amino acids of HIV’s Tat protein, reactivating latent virus and enabling infected cells to be identified for elimination.
- LNP X also successfully transported CRISPR activation machinery into cells, demonstrating potential for precise modulation of both viral and host genes.
- This targeted nanoparticle approach addresses the shortcomings of antiretroviral therapy and earlier latency-reversing agents, which often fail to shrink viral reservoirs or cause systemic toxicity.
- Further studies in animals and humans are needed to determine safety, biodistribution and how much of the latent virus must be cleared to achieve an effective cure.