Overview
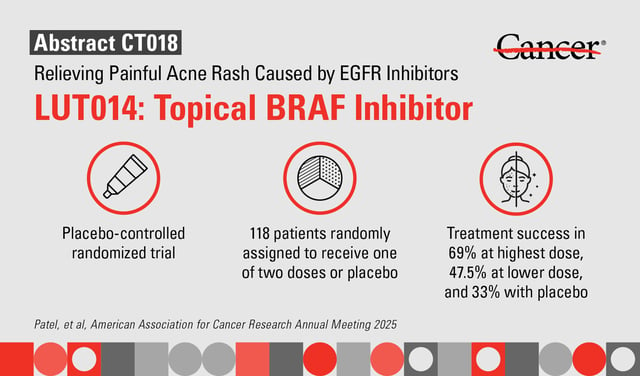
- LUT014, a topical BRAF inhibitor gel, alleviates acneiform rash caused by anti-EGFR therapies in colorectal cancer patients, improving adherence to treatment.
- In a Phase II trial of 118 patients, 69% of those using high-dose LUT014 experienced significant rash reduction compared to 33% in the placebo group.
- The gel targets the underlying mechanism of rash by reactivating the MAPK pathway locally in the skin, without interfering with systemic anti-cancer effects.
- Patients reported improved quality of life and fewer disruptions to cancer therapy, addressing a common side effect that affects 75% of EGFR inhibitor users.
- A Phase III trial is in development to further evaluate LUT014’s efficacy and explore broader applications in managing side effects of targeted cancer therapies.