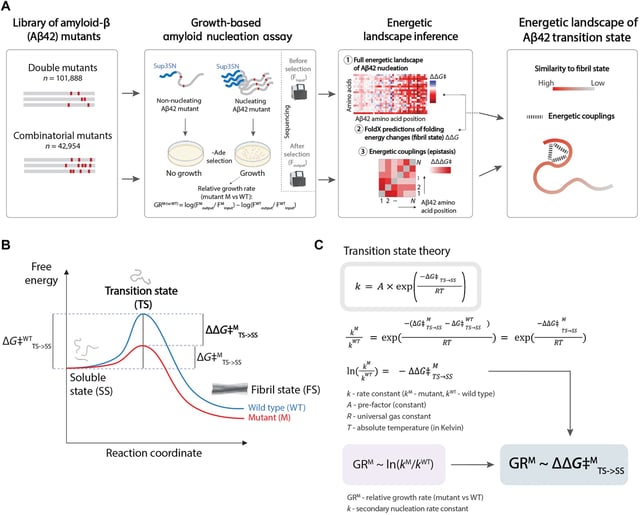
Overview
- The research team combined high-throughput DNA synthesis with yeast-based nucleation assays to quantify energy changes of individual Aβ42 mutations at scale.
- By analyzing over 140,000 peptide variants, researchers generated the first comprehensive energy landscape of the amyloid β transition state driving aggregation.
- Key interactions at the hydrophobic C-terminal region were found to exert an outsized influence on the speed of fibril growth.
- Identifying the transition-state drivers suggests that disrupting C-terminal contacts could offer a new therapeutic strategy for Alzheimer’s disease.
- The study’s scalable methodology provides a framework for probing transition states in other protein aggregation processes and diseases.