Overview
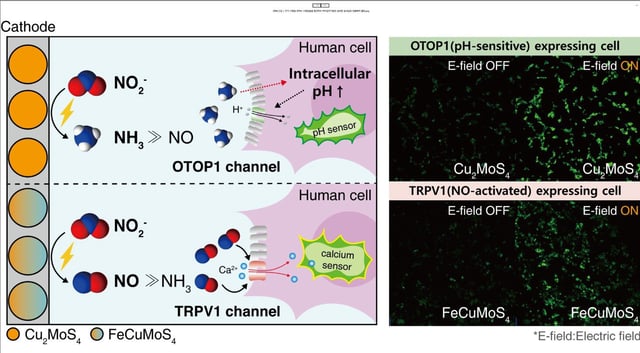
- The platform applies an electrical signal to a single nitrite precursor and switches catalysts to synthesize either nitric oxide with FeCuMoS4 or ammonia with Cu2MoS4.
- Electrochemical measurements and computer simulations reveal that iron sites in FeCuMoS4 bind nitric oxide intermediates more strongly, directing product selectivity toward NO under identical voltages.
- Human cell assays show electrochemically generated nitric oxide activates TRPV1 channels and ammonia production induces intracellular alkalinization and OTOP1 proton channel responses.
- The findings were published online in Angewandte Chemie on July 8 and formally announced by KAIST on August 11, sparking media reports that highlight its precise spatiotemporal control.
- Researchers tout the approach as a foundation for future electroceuticals, electrogenetics and personalized cell therapies, while noting that validation remains at the in vitro stage.