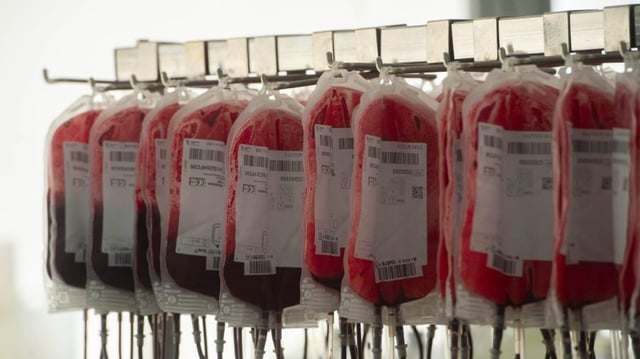
Overview
- Phase I trials at Nara Medical University began in March with escalating doses of 100 to 400 millilitres of artificial blood administered to healthy adults
- The new product encases haemoglobin from expired donor blood in a protective shell to create stable, virus-free red cell analogues with a two-year shelf life
- Universal compatibility removes the need for blood type matching and vastly extends storage compared with the current 42-day limit on donated blood
- If safety and efficacy benchmarks are met, Japan would become the first country to deploy artificial blood in real-world medical care, reshaping emergency response worldwide
- Parallel efforts in the United States, such as DARPA-funded ErythroMer, highlight growing international momentum in developing shelf-stable blood substitutes