Overview
- Tel Aviv University and spinout Matricelf say the first procedure is planned in Israel, with timing projected within about a year.
- Regulators granted preliminary authorization for up to eight compassionate-use patients, and the team says it now has approval to begin blood collection once the first candidate is cleared.
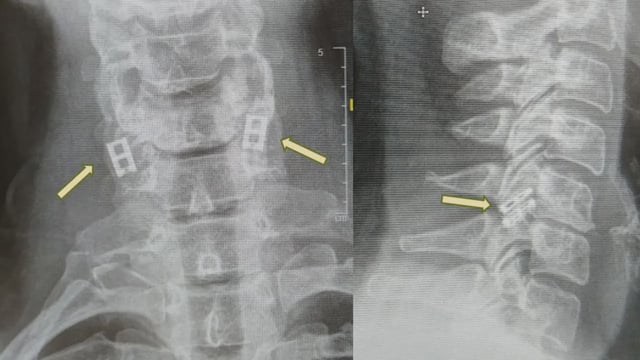
- The personalized implant is engineered by reprogramming a patient’s blood cells into embryonic-like stem cells and embedding them in a hydrogel made from the patient’s fat to form three-dimensional spinal cord tissue.
- Researchers report robust preclinical outcomes, including rats regaining normal walking and claims that more than 80% of animals recovered full mobility in earlier trials.
- Initial human candidates are expected to be those with relatively recent spinal cord injuries, and the procedures will determine safety and efficacy in people.