Overview
- An SIT from Madhya Pradesh arrested Sresan Pharmaceuticals owner G. Ranganathan in Chennai on charges including culpable homicide and drug adulteration, with transit remand sought to bring him to Chhindwara.
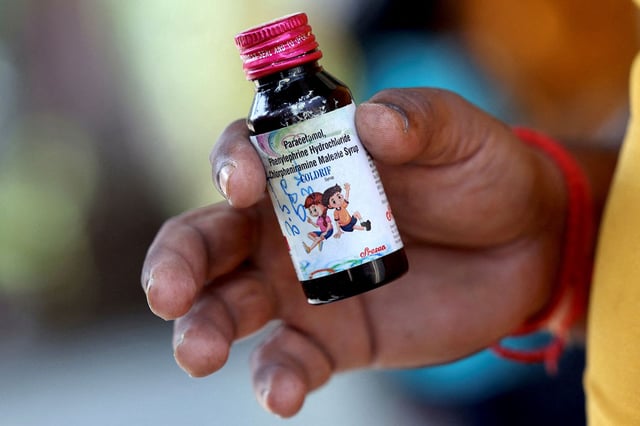
- Tamil Nadu’s government lab confirmed 48.6% diethylene glycol in Coldrif batch SR-13, a level tied to acute kidney failure in children under five.
- India’s drug regulator recommended cancelling Sresan’s manufacturing licence and ordered recalls and stop-production for Coldrif, Respifresh TR and ReLife, as multiple states imposed bans and seized stock.
- WHO requested export details and highlighted gaps in domestic DEG/EG screening, while CDSCO told the agency the identified syrups were not exported.
- Tamil Nadu sealed the Kancheepuram unit and suspended two senior drug inspectors after inspectors logged hundreds of GMP violations, as Maharashtra and other states intensified raids and sample testing.