Overview
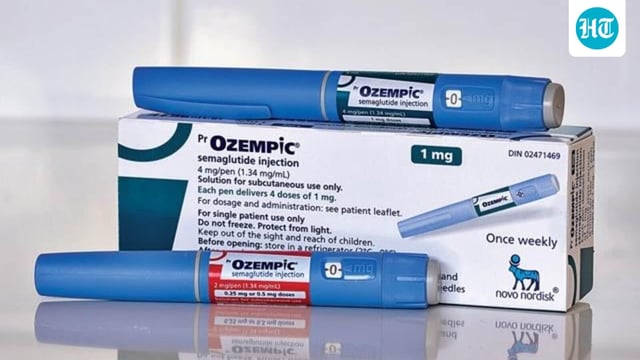
- CDSCO approved Novo Nordisk’s semaglutide injection (Ozempic) on September 26 for adults with insufficiently controlled type 2 diabetes, with public reporting on September 30.
- The Indian label specifies use as an adjunct to diet and exercise, and the product will be dispensed only on a doctor’s prescription, according to reporting.
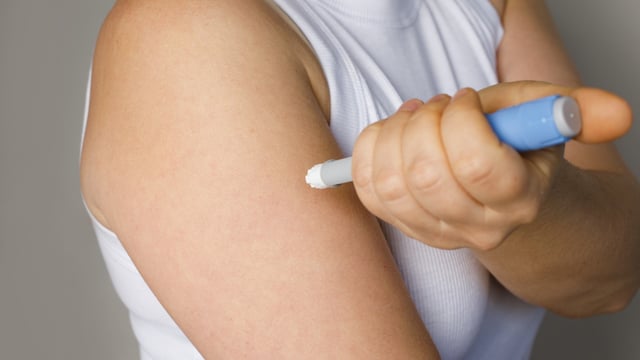
- Novo Nordisk is reported to be preparing a launch in India soon for the once-weekly shot, which mimics GLP-1 to improve insulin response and curb appetite.
- Pricing for India has not been disclosed, and market watchers expect generic entries after a semaglutide patent expiry projected in March 2026 that could lower costs.
- Coverage highlights trial-reported benefits such as HbA1c reduction, weight loss support, and cardiovascular and renal outcomes, alongside common gastrointestinal side effects including nausea.