Overview
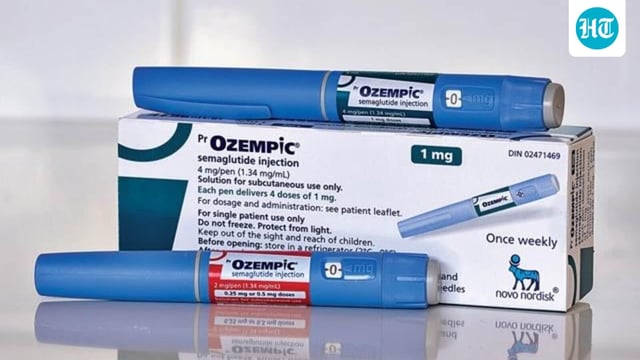
- CDSCO approved semaglutide injection Ozempic on September 26 for adults with insufficiently controlled type 2 diabetes as an adjunct to diet and exercise, with dispensing restricted to prescription use.
- Novo Nordisk has not disclosed Indian pricing and is preparing a near-term launch, with market watchers expecting initial costs comparable to existing GLP-1 and tirzepatide therapies and potential relief after semaglutide’s patent expiry in March 2026.
- Clinicians flag common gastrointestinal side effects and rare serious risks such as pancreatitis, gallbladder issues, kidney problems and thyroid warnings from animal studies, underscoring the need for medical supervision and careful dose titration.
- Experts warn against off-label cosmetic weight loss use, caution about counterfeit or black-market channels, and fear supply pressures if demand outpaces authorised distribution.
- India’s nod is for diabetes control rather than a formal weight-loss indication, even as higher-dose semaglutide (Wegovy) is licensed for obesity elsewhere and European guidelines now recommend GLP-1 drugs as first-line options for obesity treatment.