Overview
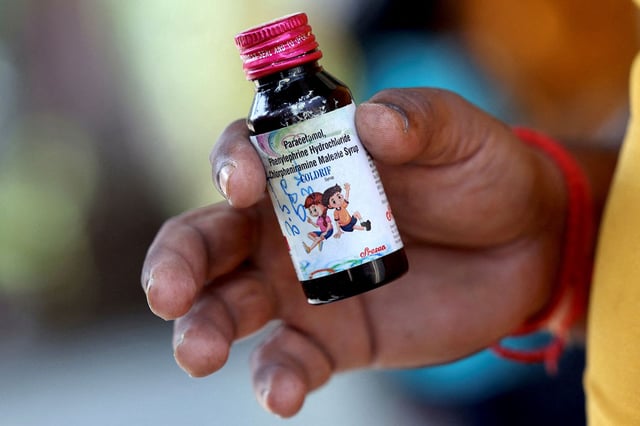
- Police from Madhya Pradesh detained Sresan Pharmaceuticals owner G. Ranganathan in Chennai, with charges including culpable homicide and drug adulteration and plans to seek transit remand to Chhindwara.
- The World Health Organization sought export details as India’s CDSCO reported recalls and halted production of three syrups—Coldrif, Respifresh TR, and ReLife—and said no official exports occurred.
- Tamil Nadu sealed the Sresan unit, issued a stop‑production order, and suspended two senior drug inspectors after an inspection documented 364 regulatory and hygiene violations.
- States including Madhya Pradesh, Tamil Nadu, Kerala and others banned or seized stocks, while central and state regulators launched wider audits and quality checks; Maharashtra’s FDA raided units and collected samples.
- Investigations continue with several children still hospitalized in Nagpur, distribution traced to Odisha and Puducherry, and cancellation of Sresan’s manufacturing licence recommended by central authorities.